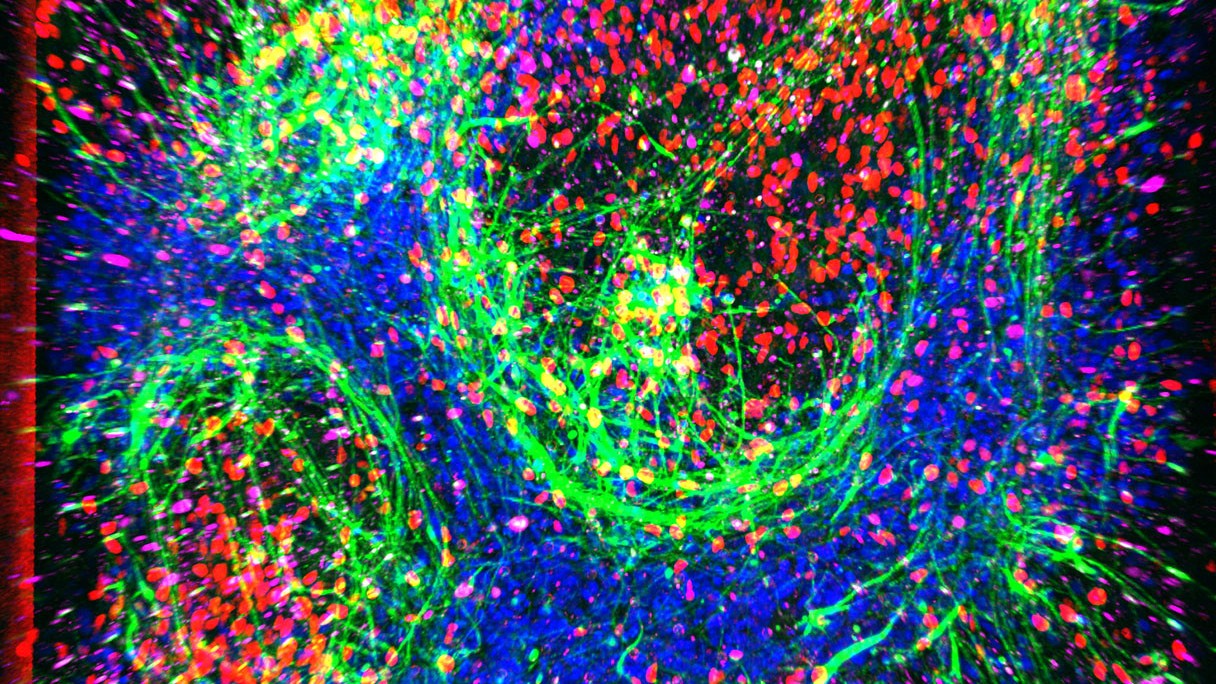
Scientists invented a pocket-sized model of the most common form of amyotrophic lateral sclerosis (ALS). The “disease-on-a-chip,” made using stem cells, could pave the way for new treatments for the progressive condition, the researchers say.
In ALS, the brain and spinal-cord cells that control voluntary muscle movements — known as motor neurons — break down and die. As a result, the brain can no longer send signals to the muscles, leading to symptoms of muscle weakness and paralysis, as well as trouble speaking, swallowing and breathing.
In a study published July 3 in the journal Cell Stem Cell, scientists unveiled a new model of sporadic ALS, which accounts for up to 95% of ALS cases and occurs spontaneously without a clear genetic cause or known family history. The platform mimics the early stages of the disease and does so more accurately than previous lab models could.
To build the model, researchers collected blood cells from young-onset ALS patients, all under age 45, and healthy male donors, whose cells were used to build a “healthy” chip, for comparison. The blood cells were reprogrammed into induced pluripotent stem cells (iPSCs), which can be turned into any type of cell in the body. The stem cells were then turned into spinal motor neurons, which normally enable movement and degenerate in ALS.
A second set of iPSCs was turned into cells similar to the blood-brain barrier (BBB), which helps prevent harmful germs and toxins from entering the brain. The spinal neurons were seeded into one channel within the chip, while the BBB cells were placed in another channel.
Separated by a porous membrane, the two chambers were then perfused with nutrient-rich fluid to mimic continuous blood flow. The resulting “spinal-cord chip” maintained both sets of cells for up to about a month and helped the neurons mature beyond what models without flowing fluids allowed.
Related: Scientists invent 1st ‘vagina-on-a-chip’
The basic chip was developed by the biotech company Emulate and then customized for use in the ALS model by researchers at Cedars-Sinai in Los Angeles, California.
Earlier models of ALS also used iPSC-derived neurons and structures mimicking those found in the brain, but they lacked dynamic flow, making it hard to capture specific aspects of the disease.
“Our previous models were static, like a dish of cells sitting still, and couldn’t differentiate between ALS and healthy cells,” said study co-author Clive Svendsen, executive director of the Board of Governors Regenerative Medicine Institute at Cedars-Sinai. “We recreated an in vitro [lab dish] environment that breathes and flows like human tissue, which allowed us to detect early differences in ALS neurons.”
Other experts agree. “Unlike most lab models that lack vascular features and dynamic flow, this chip improves neuron health and maturation,” said Dr. Kimberly Idoko, a board-certified neurologist and medical director at Everwell Neuro, who was not involved in the study. “It captures early disease signals in ALS that are often hard to detect,” Idoko told Live Science in an email.
With their ALS and healthy chips in hand, the researchers analyzed the activity of more than 10,000 genes across all the cells. One of the most striking findings was abnormal glutamate signaling in the neurons within the ALS chip.
Glutamate is a major excitatory chemical messenger, meaning it makes neurons more likely to fire and send on a message to additional neurons; its counterpart, GABA, is inhibitory. The team saw increased activity in glutamate receptor genes and decreased activity in GABA receptor genes in the motor neurons, compared to the healthy chip.
“We were intrigued to find this increase in glutamate activity,” Svendsen said. “Although there was no visible neuron death, we hypothesize this hyperexcitability could trigger degeneration at later stages.”
This finding aligns with long-standing theories about ALS, which suggest that boosted glutamate signalling contributes to nerve damage. It also corresponds with the mechanism of the ALS drug riluzole, which blocks glutamate. The new chip adds to the evidence for this mechanism and could help reveal how it manifests in the earliest stages, before symptoms would be evident in a patient, Svendsen suggested.
While Idoko praised the model, she noted it lacks glial cells — additional nervous-system cells involved in ALS — and doesn’t capture the late-stage degeneration seen in ALS. “However, a model like this could conceivably be useful for early drug screening, to study how a drug might cross a barrier similar to the blood-brain barrier, in preparation for animal or human studies,” she said.
The team is now working toward maintaining the cells in the model for up to 100 days. They also would like to incorporate other cell types, like muscle cells, to fully mimic ALS progression. As motor neurons die off in the disease, muscle cells also waste away.
“Our goal is now to build models where more neurons die, so we can better map disease pathways and test treatments in a human-like setting,” Svendsen said. For now, the chip offers a window into ALS’s earliest molecular changes and a tool to figure out how to detect and slow the disease before irreversible damage occurs.