Human stem cells get worn out and age much faster in space, a new study has found, which is a problem for anyone hoping to take a long trip through our solar system.
Scientists used artificial intelligence (AI) to track changes to stem cells delivered by SpaceX resupply missions to the International Space Station (ISS). Up in space, the stem cells lost some of their ability to generate new cells, became more susceptible to DNA damage and aged faster, according to a statement released by the researchers.
The study’s findings, published Sep. 4 in the journal Cell Stem Cell, build on previous space health research that highlight the challenges of putting people in space for prolonged periods of time — something humanity would have to overcome if it wants to colonize other planets such as Mars.
“Space is the ultimate stress test for the human body,” study co-author Catriona Jamieson, the director of the Sanford Stem Cell Institute and professor of medicine at the University of California San Diego, said in the statement. “These findings are critically important because they show that the stressors of space — like microgravity and cosmic galactic radiation — can accelerate the molecular aging of blood stem cells.”
Related: ‘Why would you even want to go?’: Readers react to the hypothetical 400-year voyage to Alpha Centauri
The human body is not built for space. Up there, our species is subjected to completely different environmental strains. Two notable, harm-causing stressors are the near-complete weightlessness of microgravity and cosmic radiation — tiny, subatomic particles that zoom through space.
Previous studies have documented that humans experience a variety of adverse health effects in space. For example, a 2022 study found that astronauts suffer decades of bone loss from spending more than six months in orbit; and scientists have even suggested that colonizing Mars may require some DNA tweaking to ensure that our bodies cope with life away from our home planet.
In the new study, researchers looked at hematopoietic stem and progenitor cells, or HSPCs. These cells regulate immune system health and cancer immune surveillance. Previous work has shown that exposure to microgravity can impact immune and metabolic changes, but not how time in space affects the molecular integrity and functional capacity of HSPCs, according to the study.
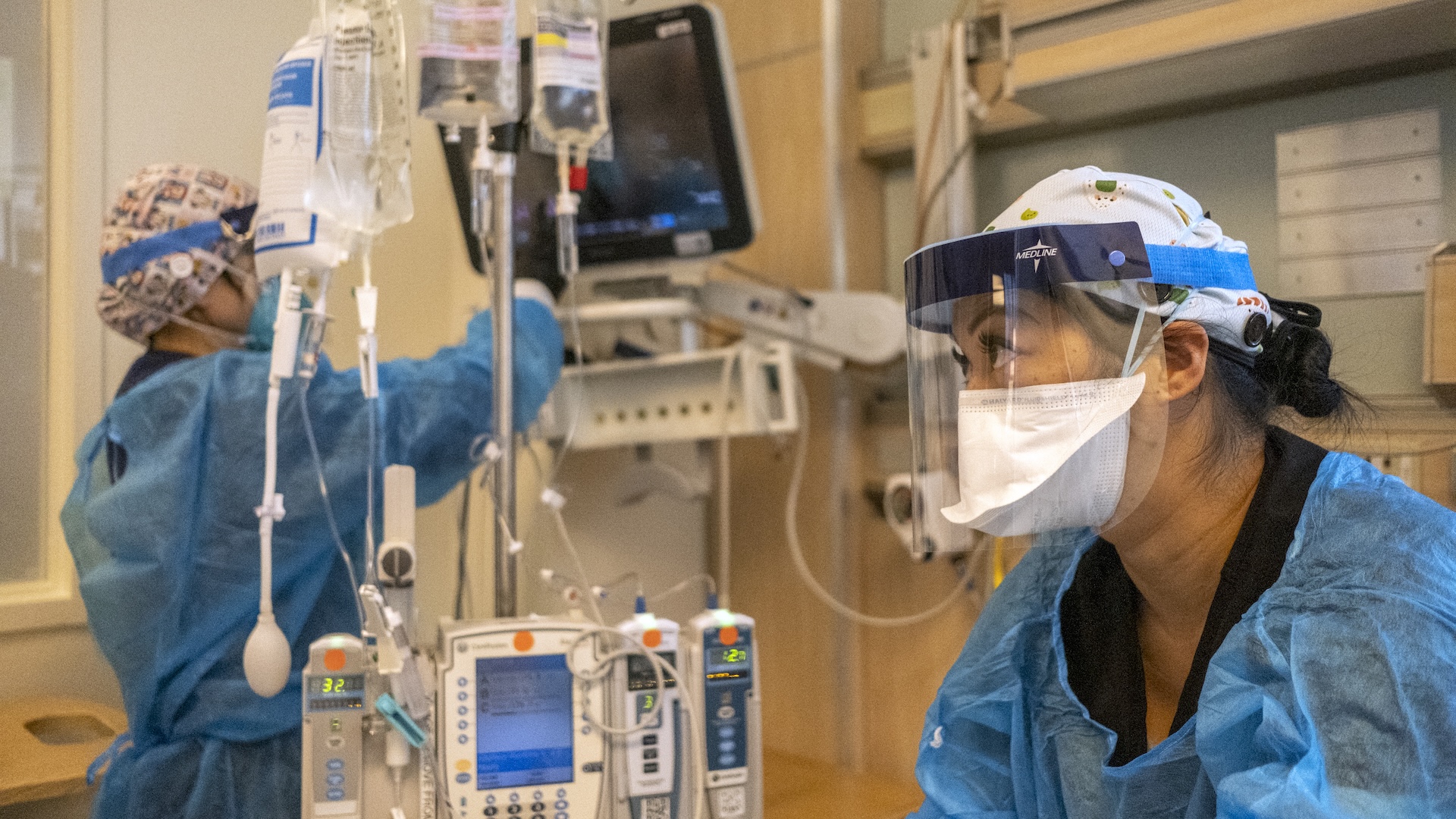
The team acquired human cells from the bone marrow of consenting individuals undergoing hip replacement procedures before growing the cells in a nanobioreactor, a type of vessel that can facilitate biological reactions. The researchers carried out their experiment on Earth and on four missions to the ISS.
Using AI-powered imaging tools to monitor cell activity, the scientists noticed that cell changes in the space samples were similar to those seen in normal cell aging on Earth, yet they occurred at an accelerated rate. For example, the cells were more active than normal and were losing their ability to rest and recover. The researchers also observed more activity in part of the “dark genome” — poorly understood regions of the genome linked to stress responses and aging, according to the study.
While the study’s findings paint an ominous picture for long-duration space travel, they do come with a silver lining: Damage seen in the cells began to reverse when they were placed in a young, healthy tissue environment. This recovery suggests that it may be possible to rejuvenate aging cells, according to the statement.
The cell changes and accelerated aging documented in the new study could help researchers better protect astronauts spending time in space, as well as people aging under normal conditions on Earth.
“Understanding these changes not only informs how we protect astronauts during long-duration missions but also helps us model human aging and diseases like cancer here on Earth,” Jamieson said. “This is essential knowledge as we enter a new era of commercial space travel and research in low earth orbit.”