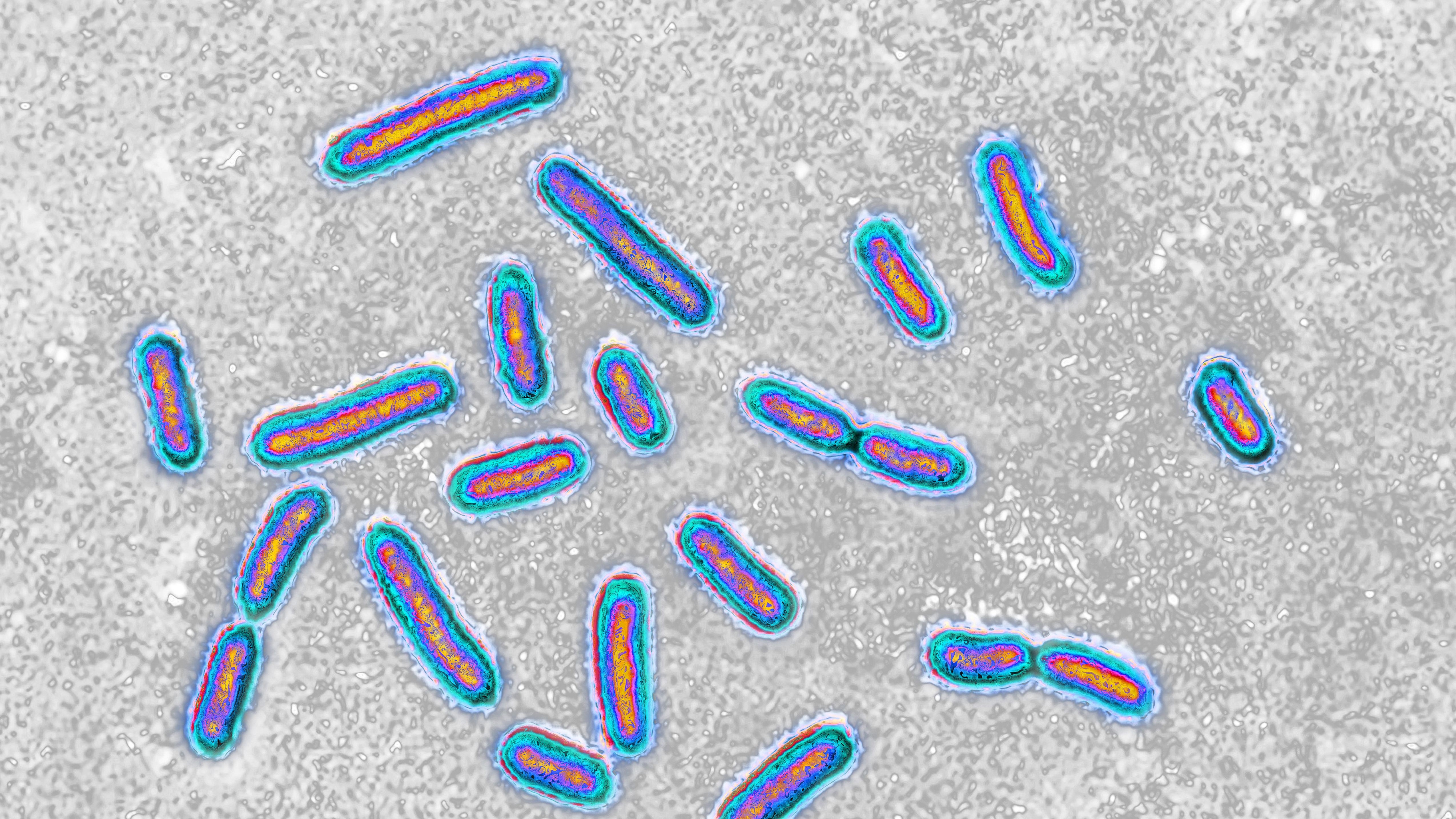
A superbug that commonly causes infections in hospitals can feed on plastic used for medical interventions, potentially making it even more dangerous, a world-first study has found.
The bug is a bacteria species called Pseudomonas aeruginosa, which is commonly found in hospital environments and can cause potentially deadly infections in the lungs, urinary tract and blood.
Now, scientists have analyzed a strain of this bacteria from a hospital patient’s wound, which revealed a surprising trick that could enable it to persist on surfaces and in patients for longer — its ability to break down the biodegradable plastics used in stints, sutures and implants. The researchers published their findings May 7 in the journal Cell Reports.
“It means we need to reconsider how pathogens exist in the hospital environment,” study lead author Ronan McCarthy, a professor in biomedical sciences at Brunel University of London, said in a statement. “Plastics, including plastic surfaces, could potentially be food for these bacteria. Pathogens with this ability could survive for longer in the hospital environment. It also means that any medical device or treatment that contains plastic could be susceptible to degradation by bacteria.”
The team’s laboratory study raises the need for further research to better understand how this plastic-eating ability affects the bug in realistic hospital environments, in which specific cleaning protocols are in place to help prevent exposing patients and medical instruments to bacteria.
P. aeruginosa is thought to have rapidly evolved over the last 200 years to infect humans as they began living in densely populated areas, especially among those with weakened lungs due to air pollution.
Related: Dangerous ‘superbugs’ are a growing threat, and antibiotics can’t stop their rise. What can?
Since then, many strains of the bug have acquired resistance to a wide variety of antibiotics. These resistant microbes can contaminate catheters and ventilation devices, making P. aeruginosa a common cause of hospital-acquired infections, especially among vulnerable patients. P. aeruginosa is tied to roughly 559,000 deaths per year globally, the majority of which are associated with antimicrobial resistance.
Yet how the bacteria can thrive in ostensibly sterile hospital environments has remained unclear.
To investigate, the researchers took a swab from a patient’s wound in a British hospital and analyzed it, which revealed the bug can make an enzyme named Pap1. This enzyme is able to break down the plastic polycaprolactone (PCL) — commonly used in sutures, wound dressings, surgical meshes and other medical equipment — and release the plastic’s carbon, which P. aeruginosa can then feed on.
To test whether this enzyme is really responsible for breaking down plastic, the scientists inserted the gene that codes for Pap1 into Escherichia coli bacteria, and found that when that bacteria expressed the enzyme, it too was able to break down PCL. The team further confirmed the enzyme’s plastic-eating role when they deleted the gene that codes for it in a P. aeruginosa variant, finding that the microbe was no longer able to dissolve the plastic.
The bug’s plastic-chewing power doesn’t just seem to be granting it a food source: It is also making it more dangerously resistant to treatment. This is because the bacteria uses plastic fragments to form hardier biofilms — structures with protective coatings that shield superbugs from antibiotics — the researchers found.
The scientists also identified similar enzymes in other bacteria, meaning that other widely used medical plastics could be providing sustenance and improved resilience to additional superbugs, possibly contributing to hospital-acquired infections.
To follow up on this, the researchers have called for urgent research on the prevalence of the plastic-eating enzymes among other pathogens, and for experts to reconsider the plastics they use in medical settings, and the ways that they monitor hospital environments.
“Plastic is everywhere in modern medicine, and it turns out some pathogens have adapted to degrade it,” McCarthy said. “We need to understand the impact this has on patient safety.”
This article is for informational purposes only and is not meant to offer medical advice.