Scientists have found that twisting structures in DNA long mistaken for knots are actually something else entirely.
Inside cells, DNA gets twisted, copied, and pulled apart. The twists can influence how genes function, affecting which are switched on and when. Studying how DNA responds to stress can help scientists better understand how genes are controlled, how the molecule is organized, and how problems with these processes might contribute to disease.
For years, researchers have been using nanopores — tiny holes just wide enough for a single DNA strand to slip through — to read DNA sequences quickly and inexpensively. These systems work by measuring the electrical current flowing through the nanopore. When a DNA molecule passes through, it disrupts that current in a distinct way that corresponds with each of the four “letters” that make up DNA’s code: A, T, C and G.
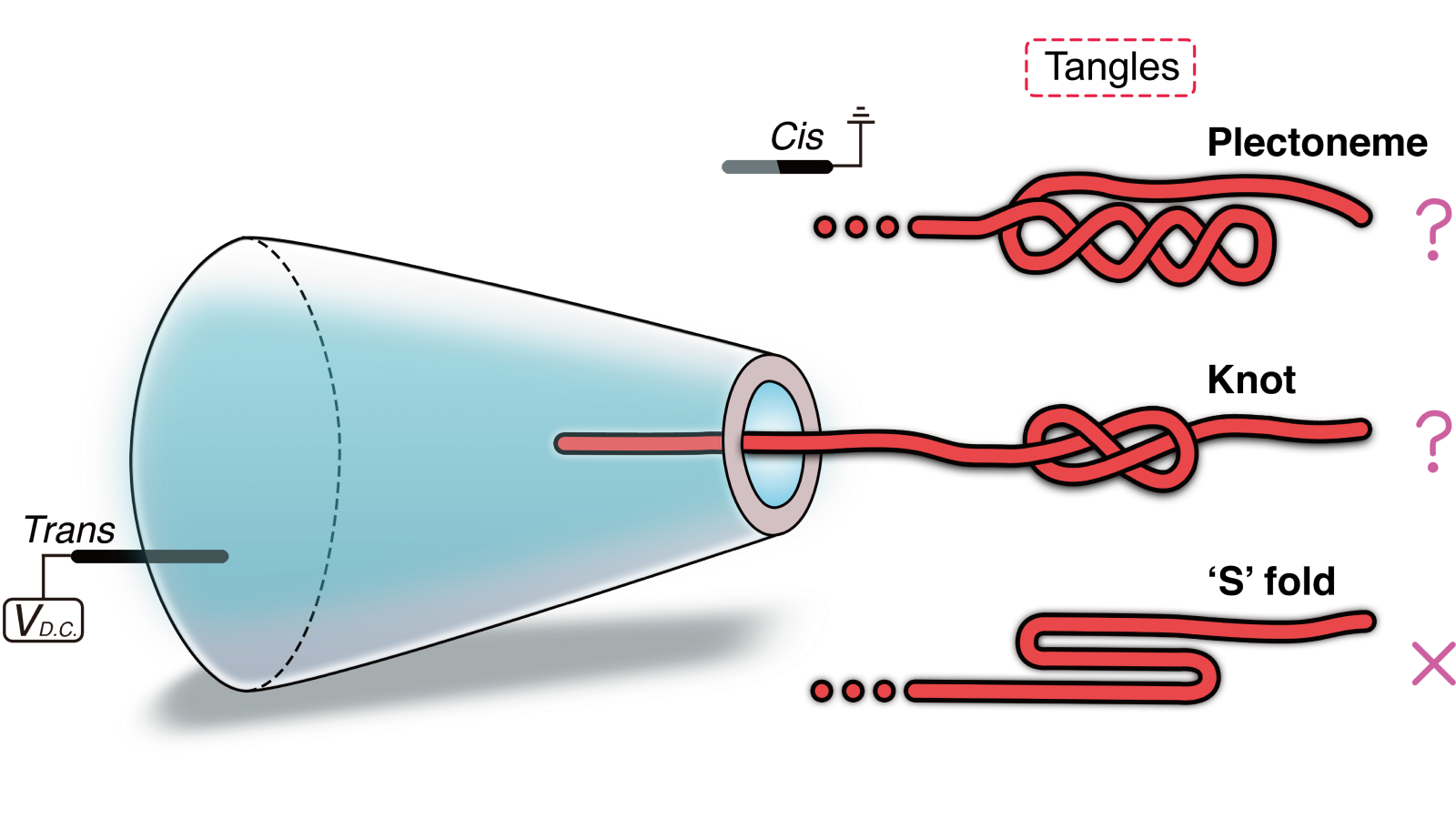
Unexpected slowdowns or spikes in this signal were often interpreted as knots in DNA. But now, a new study published Aug. 12 in the journal Physics Review X finds that these signal changes can also signify plectonemes, which are natural coils that form when DNA twists under stress.
“Knots and plectonemes can look very similar in nanopore signals,” lead study author Ulrich Keyser, a physicist at the University of Cambridge’s Cavendish Laboratory, told Live Science. “But they come from very different physical mechanisms. Knots are like tight tangles; plectonemes are more like coiled springs, formed by torque.”
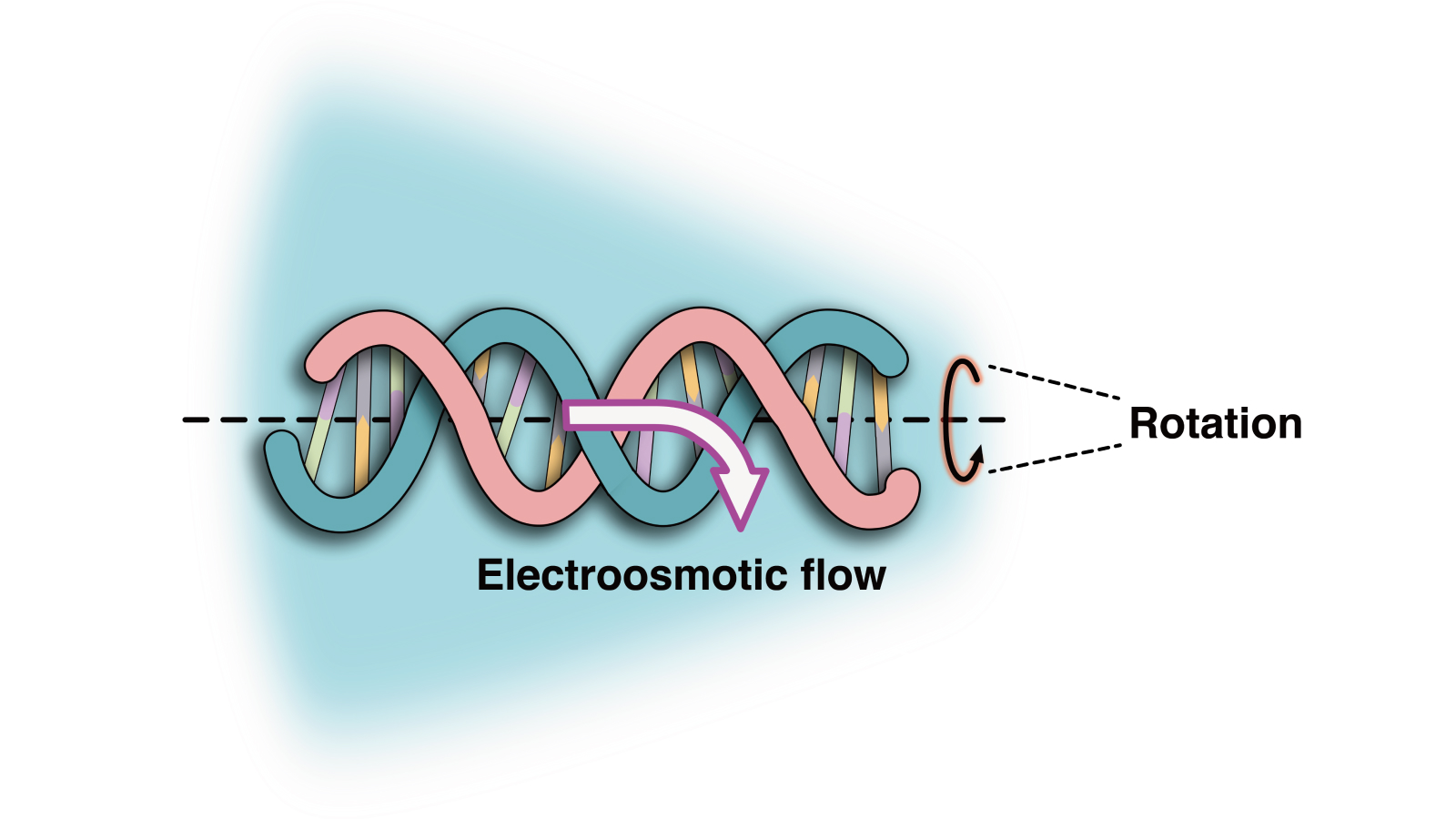
To study these coils, the researchers passed a DNA strand through a cone-shaped nanopore in a salty solution with a high pH. The solution helped to create an electroosmotic flow, meaning the DNA began to spin as it entered the pore. The motion generated a strong enough twisting force, or torque, that it coiled the DNA, Keyser explained.
Related: DNA has an expiration date. But proteins are revealing secrets about our ancient ancestors we never thought possible.
Keyser and his team also applied an electrical voltage across the nanopore to help drive the DNA through and measure changes in electrical current.
“In these kinds of nanoscale systems, everything is very high friction, so the DNA moves almost like it’s swimming through honey,” Keyser said. “It’s a very viscous environment, so relatively high forces push the DNA in this corkscrew motion.”
The researchers analyzed thousands of these events. While some knots still appeared in the experiment, they tended to be smaller — approximately 140 nanometers across — whereas plectonemes were about 2,100 nanometers across. As the voltage applied to the system was increased, plectonemes became more common due to a stronger torque.
To further test how twisting affects DNA behavior, the researchers introduced small breaks, called nicks, into one strand of DNA’s double helix. These nicks enabled the DNA to rotate more easily and release built-up tension, which, in turn, caused fewer plectonemes to form. This confirmed that torsional stress is a key driver of these structures’ formation.
“When we controlled the molecule’s ability to rotate, we could change how often plectonemes appeared,” Keyser said.
Although nanopores are very different from living cells, these kinds of plectonemes may also form during processes like DNA transcription and replication. Transcription describes when DNA’s code gets copied down by another molecule, called RNA, and shipped off into the cell. Replication describes when the DNA molecule is replicated in full, which happens when a cell divides, for instance.
“I believe that the torsion in the molecules can actually give rise to the formation of i-motifs and G-quadruplexes,” Keyser told Live Science, giving the names of two specific types of knots seen in DNA. So what they found in their lab study likely has implications for living cells, he explained.
Keyser and his team have been investigating how plectonemes and other DNA structures form during natural processes, such as transcription. In earlier work, they explored how torsional stress affects DNA replication. Nanopores give scientists a way to not only read DNA but also to watch how it behaves, this study emphasizes.
“Just the fact that the DNA molecule can squeeze through the pore, where its stiffness is supposed to be much larger than the pore diameter, is quite amazing,” Slaven Garaj, a physicist at the National University of Singapore who was not part of the study, told Live Science. “It’s 10, 50, even 100 times stiffer than the pore size. Still, it bends and passes through.”
Garaj was excited about the findings. In the future, “we might be able to separate nanopore-induced torsion from torsion that was already in the DNA before. That could let us explore natural supercoiling in new ways,” he added. This would be important for understanding how coils and knots control gene activity.