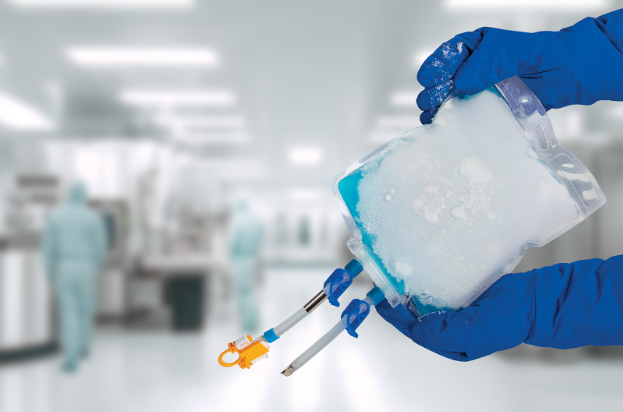
Meissner has expanded the capabilities of its TepoFlex® biocontainer platform to include freeze and thaw applications, extending a proven single-use solution into workflows that demand exceptional material strength and reliability.
The robust performance of the TepoFlex® polyethylene film platform has been validated through almost 20 years of industry use. Applying these established strengths, the biocontainers are engineered to withstand the stresses of freeze and thaw cycles, supporting secure handling of drug substances and drug products during storage, transport and processing.
The 2D TepoFlex® biocontainers are suitable for freezing at temperatures as low as -80°C. Available as single-use containers in volumes from 50 mL through to 10 L, they provide flexibility for a wide range of biopharmaceutical applications and process scales.
The containers’ flexible design allows seamless integration into existing freeze and thaw systems. Compatibility with conventional blast freezers and vertical plate freezers enables manufacturers to adopt the solution without major changes to infrastructure or additional capital expenditure.
For manufacturers seeking dependable single-use solutions to safeguard sensitive materials under low-temperature conditions, the TepoFlex® biocontainer offers a reliable and adaptable option.
All freeze and thaw biocontainers are manufactured in ISO-certified cleanrooms using qualified materials to support biocompatibility and regulatory compliance. In addition, Meissner’s Applications Engineering Team offers expert guidance to help design and optimise single-use systems tailored to specific operational needs.
With more than 40 years of experience supporting the biopharmaceutical industry, Meissner places strong emphasis on collaboration and customer satisfaction, working closely with clients to understand challenges and support long-term success.
To discover why leading biopharmaceutical manufacturers trust TepoFlex® for their most essential freeze and thaw applications, visit Meissner’s website at https://www.meissner.com/tepoflex-freeze-thaw where technical resources are available for download.
Additionally, visiting https://www.meissner.com/applications/freeze-thaw/ provides an overview of all of Meissner’s single-use freeze and thaw platform offerings.
About Meissner
Meissner manufactures advanced microfiltration products and therapeutic manufacturing systems for critical pharmaceutical and biopharmaceutical applications, such as sterilization of injectable drugs, and the development and manufacture of life-enhancing/life-saving drugs, therapeutics, biologics, and cell and gene therapies.
Meissner’s manufacturing campuses include a headquarters location in Camarillo, California, USA, a European manufacturing location in Castlebar, Ireland, and buildout of a $250 million facility in Athens, Georgia.
Meissner’s product portfolios are built upon a solid foundation of quality, operational excellence, and technical expertise for delivery of high-performance products that support clients’ critical applications. For more information about Meissner, please visit https://www.meissner.com.