The bacteria behind chlamydia can colonize the gut, and from that hiding place, they may act as a source of repeated infections, new research using miniature intestines suggests.
Chlamydia is the most common sexually transmitted infection (STI) worldwide. The form of the infection that affects humans is caused by a species of bacteria known as Chlamydia trachomatis.
The disease most often affects the genital region, sometimes causing pain and unusual discharge from the vagina or penis. However, over the years, research in mice and various clinical reports in humans have suggested that C. trachomatis may also be able to infect the human digestive tract. This means that, theoretically, the bacteria could hide in the gut and then cause repeated genital infections, which commonly occur in patients despite treatment with antibiotics.
Yet, until now, scientists haven’t been able to test this theory in human cells.
Related: As syphilis levels hit 70-year high, sexually transmitted infection epidemic shows ‘no signs of slowing’
Now, in a new study published Thursday (Aug. 22) in the journal PLOS Pathogens, researchers used miniature, lab-grown models of different parts of the human digestive tract to study whether C. trachomatis could indeed infect the gut.
To make these models, the team used chemicals to coax stem cells taken from adult donors to grow into miniature, 3D replicas of full-size organs. These included the large and small intestines. These types of tiny models, known as “organoids,” have become an increasingly popular tool for scientists to study physiological systems and to test new drugs. Organoids can be made to more accurately recapitulate the structure and function of human organs, compared with other cell-based or animal models.
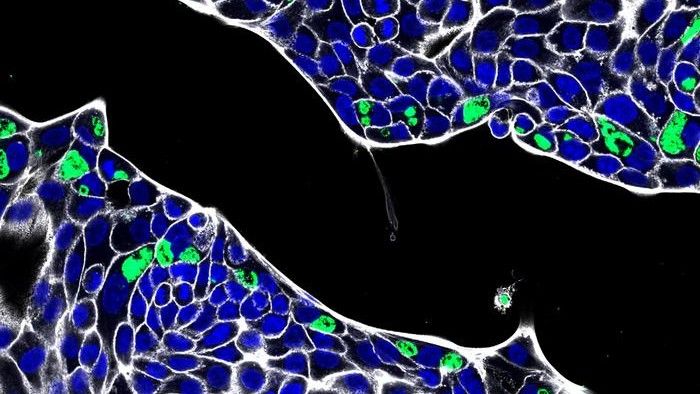
Once the digestive organoids had grown to a large enough size, the researchers extracted specific cells called primary intestinal epithelial cells, which line the surface of the intestines. They then grew these cells in single layers in a lab dish, to expand their numbers, before infecting them with C. trachomatis. They zoomed in on this interaction under a high-resolution microscope.
The researchers showed that C. trachomatis was able to enter the cells. In some cases, the development of the bacteria was restricted, so instead of causing a full-blown infection, C. trachomatis formed large, irregularly-shaped structures called aberrant bodies. These aberrant bodies are thought to be able to persist within host cells in the body.
“This is the first report of C. trachomatis infection in human primary intestinal epithelial cells,” the study authors wrote in their report. This supports the idea that there’s a “possible niche for chlamydial infection in the human intestinal tissue,” they proposed.
In a separate experiment, the team also showed that C. trachomatis relied on a ringlet of DNA, known as a plasmid, to infect and grow within epithelial cells. This plasmid is known as Pgp3 and is made by the bacteria itself. After trying and failing to infect cells with modified strains of the bacteria that didn’t carry Pgp3, the team realized the microbe’s reliance on the plasmid.
The team acknowledged several limitations of the study, including that they looked only at how C. trachomatis infects isolated epithelial cells, rather than those embedded in an organoid. There are many factors in the human gut, such as other microbes and cells of the immune system, that could help thwart infection of these cells in real life, they noted. Therefore, it’s still not completely certain that these infections happen in human patients.
Nevertheless, the models that the scientists created should help facilitate future research into the possible role of chlamydia infection in the gut, the team said.
Ever wonder why some people build muscle more easily than others or why freckles come out in the sun? Send us your questions about how the human body works to community@livescience.com with the subject line “Health Desk Q,” and you may see your question answered on the website!