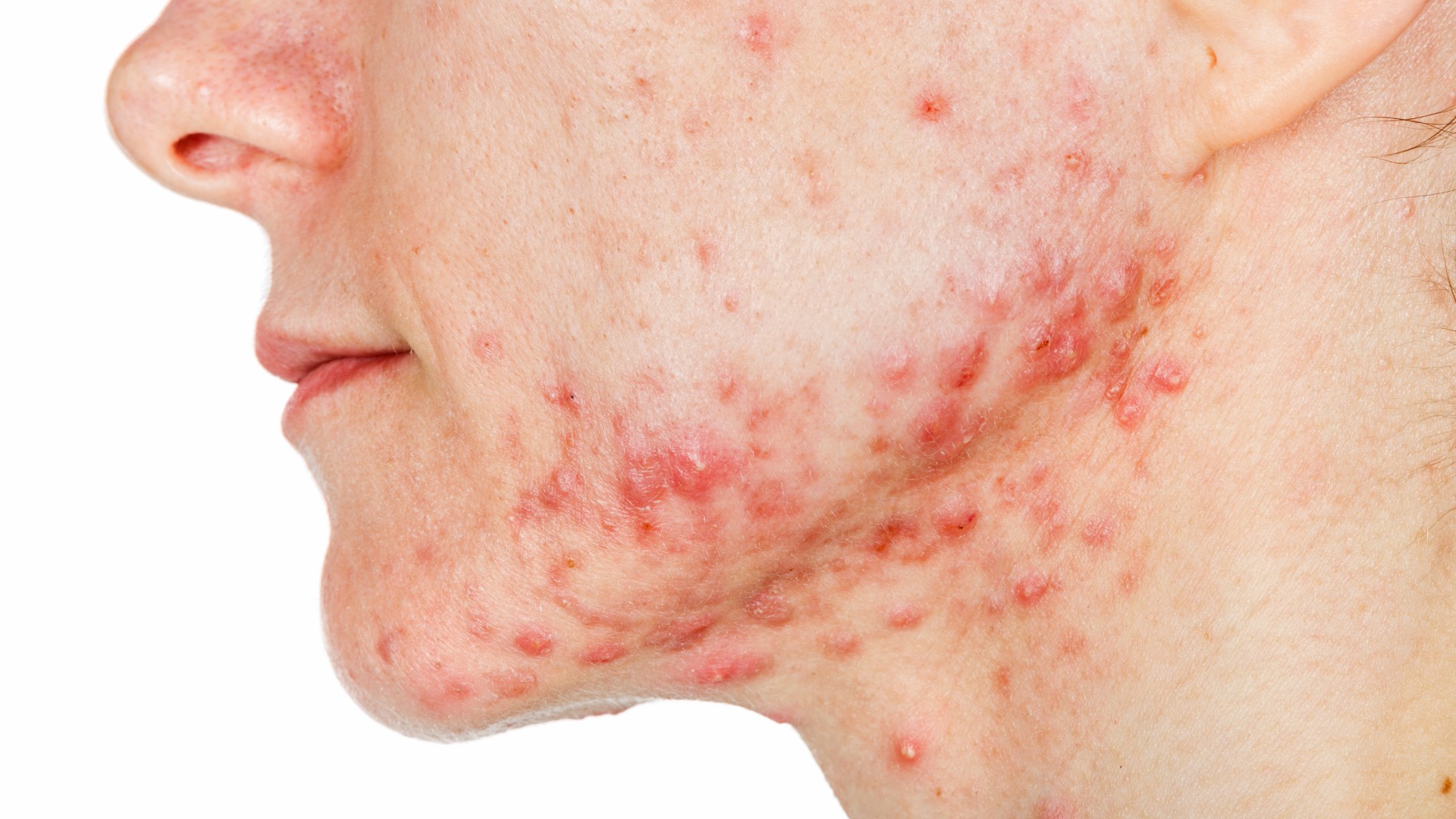
Scientists are testing a vaccine to treat acne, a skin condition that affects around 95% of people between the ages of 11 and 30. If the shot makes it through trials, it could become the first-ever acne vaccine.
Acne is an inflammatory disorder that causes hair follicles and pores in the skin to become clogged, leading to the formation of pimples that most commonly appear across the face, upper arms, trunk and back. The condition can have numerous triggers, including a sensitivity to certain body-made hormones, especially those that shift in adolescence; taking specific medications; and carrying certain genetic factors. Bacteria on the skin, such as the species Cutibacterium acnes, can also contribute to acne.
Now, the pharmaceutical company Sanofi is running an early-stage clinical trial to test the safety and efficacy of a vaccine for adults with moderate to severe facial acne.
The new vaccine could “help reshape the acne treatment landscape,” a Sanofi spokesperson told Live Science via email. Indeed, a vaccine like this one could offer a promising alternative to current acne treatments that are not curative, must be used for a long time and often come with unpleasant side effects. Such treatments include retinoids, antibiotics and hormonal contraceptives.
Related: When not causing breakouts, acne bacteria may strengthen the skin’s protective barrier
However, the trials of the new vaccine are still in their initial stages, and no data from the trials are publicly available yet to confirm whether it really works.
Here’s what we know about the experimental vaccine so far.
What is the Sanofi acne vaccine trial?
Sanofi is conducting what’s known as a Phase I/II trial. It began in April 2024 and is expected to run until 2027. During that time, the company is planning to recruit around 400 adults ages 18 to 45 who have moderate to severe facial acne, as defined by having a specific number of pimples on their face.
Some participants in the trial will receive one of three doses of the vaccine; these participants will be injected up to three times with that dose over the course of the trial. Meanwhile, other trial participants will instead receive a “dummy” vaccine that doesn’t contain any medicine. This will give the scientists a comparison point to help them determine how safe and effective the vaccine is.
How does the Sanofi vaccine work?
In a statement to Live Science, Sanofi didn’t disclose any details about how the new vaccine actually works.
However, details of the trial that were shared online note that it is an mRNA vaccine. That means it uses a genetic molecule called messenger RNA to deliver instructions into cells of the body; once in the body, the vaccine prompts the immune system to attack specific proteins.
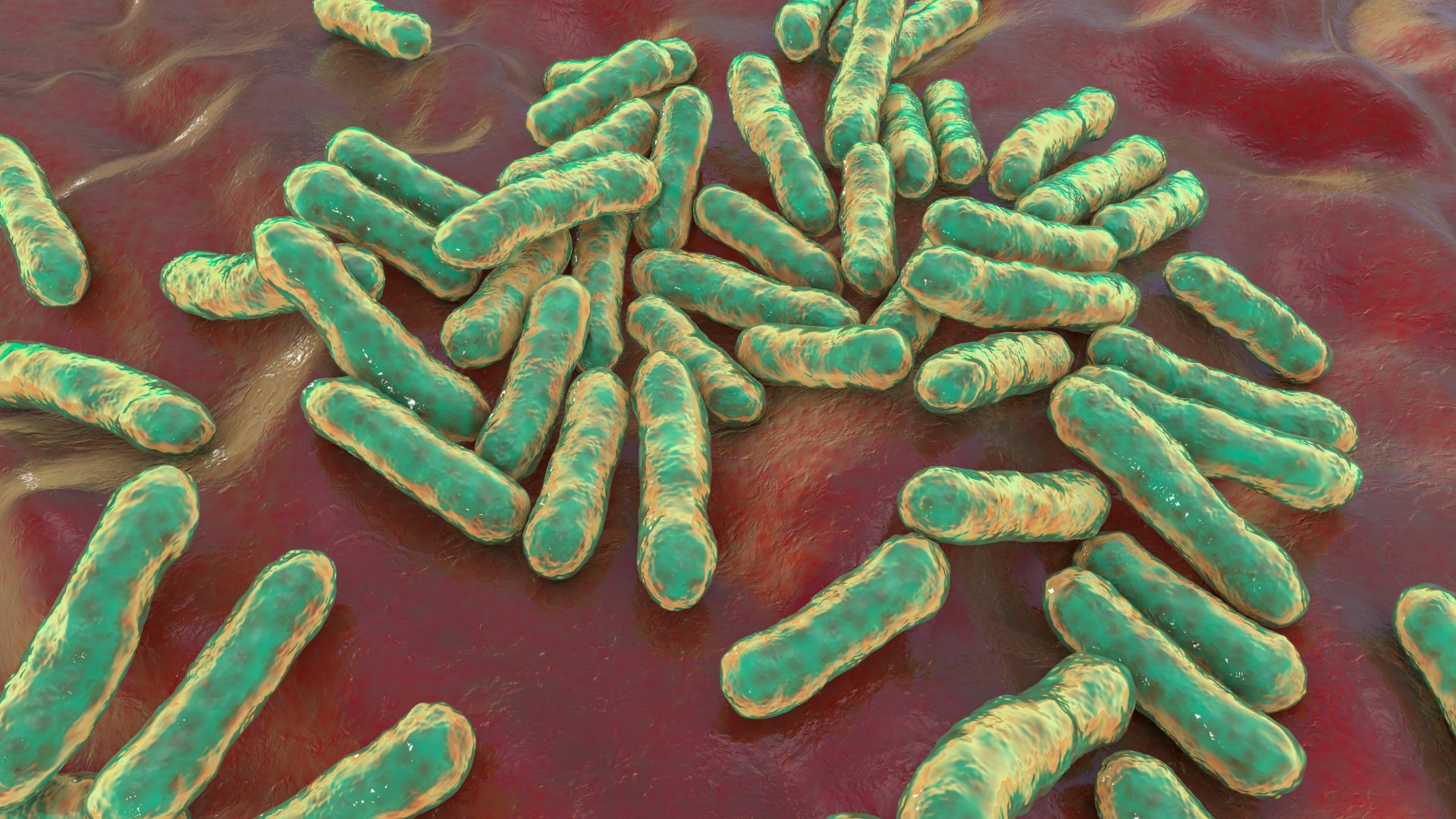
In this case, the target proteins are likely to be proteins made by C. acnes, as Sanofi told Live Science that the vaccine aims to boost a patient’s immune response against specific strains of bacteria thought to contribute to acne development. C. acnes is the primary bacterium associated with acne.
Why is there a need for an acne vaccine?
Various treatments can help manage the symptoms of acne. Such treatments target the different drivers of acne, such as the heightened sensitivity of oil glands in the skin to hormones or the bacteria that trigger inflammation.
For example, these treatments include antibiotics, which aim to control the bacterial populations on the skin, and retinoids, which increase skin cell production and thus help unclog pores. Topical antibiotics and retinoids can be applied directly to the skin in the form of lotions or moisturizers. There are also oral antibiotics and retinoids that are taken by mouth. Contraceptive drugs can help curb acne by modulating sex hormones in the body.
However, these treatments can only help control acne, rather than combatting the underlying causes of the condition. They can cause some unwanted side effects; for instance, taking retinoids can lead to skin dryness and irritation, and has been tied to psychological symptoms, such as depression and suicidal thoughts.
There is also a risk that overusing antibiotics can lead to the development of antibiotic resistance in acne-causing bacteria, meaning the microbes would partially or completely stop responding to common drugs. This problem is exacerbated by the fact that the antibiotics for acne often need to be taken over many months.
Therefore, Sanofi argues that there’s a considerable need for new treatment options, which could be afforded by a vaccine — a sentiment that has been echoed by other experts.
Related: Can sugar cause acne?
When might the Sanofi vaccine be available to patients?
Sanofi says it intends to share results from the Phase I/II trial “in due time.” Data collection for the trial is scheduled to end in 2027, so it would likely be sometime after that.
It usually takes around a decade for a vaccine to go from being designed to being licensed and approved for widespread use. Candidate vaccines have to go through multiple testing phases, starting with experiments in animals and human cells, before moving into progressively larger clinical trials with human subjects.
Even if this initial safety and efficacy trial provides positive results, much more testing will be required before the vaccine could reach clinics.
Many questions about the vaccine will need to be answered, including how often it would need to be administered, how long any beneficial effects last, and whether it could be used as a preventive therapy, rather than just a treatment for acne that’s already emerged.
Sanofi is also planning to launch a separate Phase I clinical trial of the vaccine in 2027. In this trial, the company will test how effective the vaccine is at treating patients with milder forms of acne.
Are there any other acne vaccines in the pipeline?
Sanofi scientists are not the only ones developing an acne vaccine, although they do appear to be the farthest along the development pipeline.
For instance, a group of researchers in California has created a vaccine that targets a variant of a specific enzyme known as hyaluronidase in C. acnes. This variant — which is only produced by C. acnes bacteria that cause acne — partially breaks down hyaluronic acid, a protective substance naturally produced by skin. This leaves behind fragments of the acid that the immune system then attacks, triggering the inflammation seen in acne.
In mice, this vaccine has been shown to reduce the severity of acne by 50%, compared with rodents that were not given the vaccine. Off the back of this success, the researchers are now looking to usher the vaccine into clinical trials, Dr. George Liu, a professor of pediatrics at the University of California, San Diego, who helped develop the vaccine, told Live Science.
Liu said it would be “really exciting” to contribute to helping patients who have suffered considerably from acne. However, he cautioned that a really effective vaccine would need to address factors other than bacteria that drive the condition.
This article is for informational purposes only and is not meant to offer medical advice.